
Swipe left for English· 提名Kv1.3抑制剂DPT0218作为治疗IBD和AD等多种免疫疾病的临床前候选化合物;· DPT0218是一种口服、高活性和高选择性抑制剂,具有高肠道暴露、极低系统暴露的药代特征,在体内/外的临床前研究中都展现出极佳的安全性;· 该项目再次验证了深势科技RiDYMO®药物研发平台的能力。
2024年1月30日,深势科技药物研发团队宣布提名一款靶向Kv1.3的口服高选择性小分子抑制剂DPT0218为临床前候选化合物,用于治疗多种免疫性疾病,包括炎症性肠病(Inflammatory Bowel Disease, IBD)和特应性皮炎(Atopic Dermatitis, AD)等。Kv1.3通道是一种电压门控的钾离子通道,其高表达对于维持诱导炎症反应所需的T细胞膜电位至关重要[1]。Kv1.3增强钙调神经磷酸介导的免疫细胞的激活和增殖[2],因此抑制Kv1.3可以特异性地损害炎症免疫细胞,而对机体的正常免疫功能无影响。2022年6月,制药巨头礼来公司以支付D. E. Shaw Research (DESRES)公司总价5.35亿美金、首付6000万美金的价格,获得其Kv1.3抑制剂DES-7114在全球的临床开发和商业化权利。最近,T细胞的活性已经成为治疗IBD的一个令人信服的干预点[3]。长寿的记忆T细胞群,特别是组织驻留亚群,是导致IBD的复发和持续发作的关键因素[4],而Kv1.3在记忆T细胞中表达上调并在维持其活性上发挥核心作用。据报道,与健康志愿者相比,IBD患者外周血和肠粘膜中T细胞的Kv1.3表达均显著上调[5]。据此,Kv1.3是干预T细胞活性、治疗IBD最有潜力的靶点。据估计,全球有不少于800万的IBD患者,其中欧美IBD患者已超过500万,目前IBD患者仍有着巨大的未满足临床需求。据预测,2028年全球自免药物市场规模将达到1400亿美元,其中IBD药物市场规模将达到280亿美元,占据20%的市场份额。DPT0218是一款潜在“Best-in-Class”的高选择性Kv1.3小分子抑制剂,具有新颖的分子骨架,已经在IBD、AD等数种自身免疫性疾病的临床前动物模型中展示出优越的效果。DPT0218活性在亚纳摩尔级别,对Na+、K+、Ca2+等多种离子通道实现了2000倍以上的选择性,且具有高肠道暴露、极低系统暴露的药代特征,在体内/体外的临床前研究中都展现出了极佳的安全性。深势科技首席药物官张晓敏博士表示:“抗体大分子在治疗中重度IBD中扮演着重要角色,但其存在疗效受限、免疫原性、肠外给药、耐受性相对差、成本高等缺陷,而新型口服小分子药物的出现可以解决抗体的局限性。我们的研究表明,DPT0218在体内和体外研究中均能显著抑制T细胞的激活、增殖和细胞因子分泌,从而成为潜在治疗IBD的有效手段。我们将积极推进这款PCC分子进入临床研究,并将拓展DPT0218在其它自免适应症上的应用。”深势科技创始人兼CEO孙伟杰表示:“深势科技坚持‘AI for Science’ 科学研究范式,致力于将‘AI+分子模拟+实验’落地于实际临床需求。离子通道的动力学过程极其复杂,RiDYMO®平台无疑正适合应用于这一系列高难度体系的药物开发。DPT0218的成功发现是达成AI for Science愿景的重要一步。我们期待与该领域经验丰富的药企建立合作关系,共同推动这个项目进入下一个里程碑。”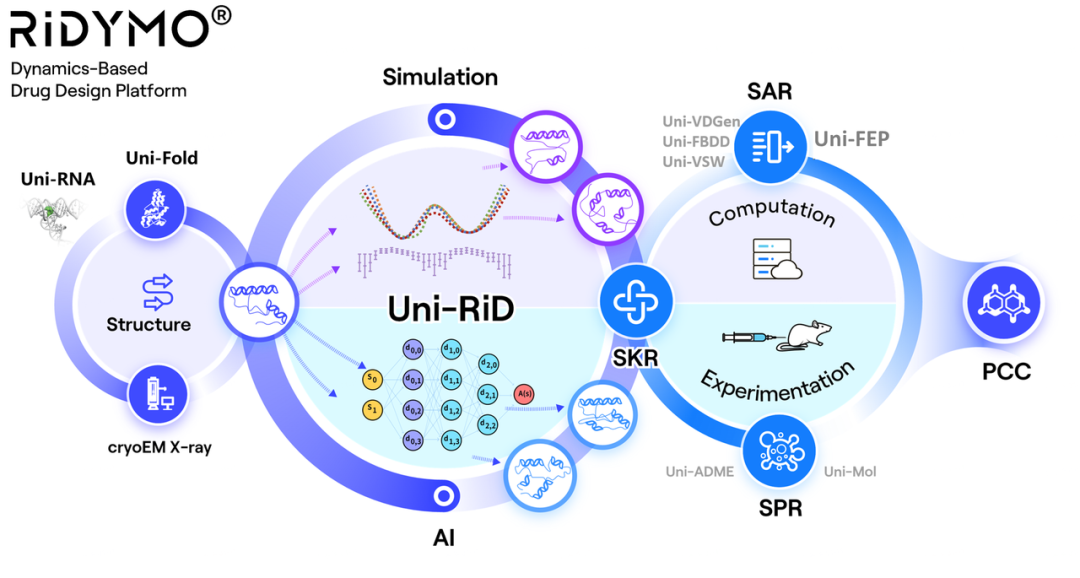
RiDYMO®药物研发平台融合了多种AI及物理模型算法,致力于“难成药”靶点及“Best-in-Class”分子的药物开发。作为其核心算法之一的强化动力学算法(Reinforced Dynamics,RiD),在分子模拟采样效率上具有显著优势。通过充分结合神经网络的高维表示能力,RiD能够在复杂的生物大分子体系中高效地捕捉动态构象变化。
RiDYMO®平台致力于研究生物体系的复杂动力学及揭示隐藏的结合位点,包括以下一系列具有挑战性的体系:protein-protein interactions (PPIs), intrinsically disordered proteins (IDPs), membrane proteins, RNA等。此前在难成药靶点c-Myc(c-Myc新突破!深势科技RiDYMO®动力学平台助力FIC药物发现)和GPX4(动力学+铁死亡:深势科技首次公开RiDYMO®平台管线应用进展)上也有所验证。
关于深势科技药物研发团队
深势科技药物研发团队基于“AI for Science”新范式,专注于利用人工智能和分子模拟相融合的先进计算手段驱动难成药靶点的药物开发。团队由张晓敏博士领衔,核心成员具备深厚的交叉学科背景和丰富的药物开发经验,同时依托深势科技全球领先的强化动力学平台RiDYMO®和大分子从头设计平台,目前已通过“自建管线+联合开发”的方式形成了完整的研发体系,覆盖CNS、肿瘤和自身免疫性疾病三大领域。
更多信息欢迎访问:https://www.dp.tech/商务合作请联系:bd@dp.tech媒体垂询请联系:pr@dp.tech
参考资料
[1] Chiang EY, Li T, Jeet S, et al. Potassium channels Kv1.3 and KCa3.1 co-operatively and compensatorily regulate antigen-specific memory T cell functions. Nat Commun 2017:14644.
[2] Lin CS, Boltz RC, Blake JT, et al. Voltage-gated potassium channels regulate calcium-dependent pathways involved in human T lymphocyte activation. J Exp Med 1993:637–45.
[3] Sandborn WJ, Su C, Sands BE, et al.; OCTAVE Induction 1, OCTAVE Induction 2, and OCTAVE Sustain Investigators. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017:1723–36.
[4] John T. Chang. Pathophysiology of Inflammatory Bowel Diseases. N Engl J Med 2020:2652-64.
[5] Anna-Lena Unterweger, Morten Ø. Jensen, Fabrizio Giordanetto, et al. Suppressing Kv1.3 Ion Channel Activity with a Novel Small Molecule Inhibitor Ameliorates Inflammation in a Humanised Mouse Model of Ulcerative Colitis. Journal of Crohn’s and Colitis 2021:1943–58.
右滑查看中文版
· The nomination of novel Kv1.3 inhibitor DPT0218 for the treatment of various immunological diseases including IBD and AD. · DPT0218 is a gut-restricted, highly potent and selective Kv1.3 inhibitor with excellent in vivo efficacy and safety profile in preclinical studies.
· This project further validates the capabilities of DP Technology’s drug design platform RiDYMO®.
DP Technology, an “AI for Science” new paradigm-driven company, today announced the nomination of DPT0218, a novel small molecule targeting Kv1.3, as a preclinical candidate compound for the treatment of various autoimmune diseases, including Inflammatory Bowel Disease (IBD) and Atopic Dermatitis (AD).The voltage-gated potassium channel Kv1.3 is known to be crucial for maintaining the membrane potential required for the induction of inflammatory responses[1] because Kv1.3 inhibition reduces calcineurin mediated immune cell activation and proliferation[2]. Inhibition of Kv1.3 therefore offers a potential opportunity to specifically impair inflammatory immune cell activity and proliferation, including in antigen-activated T cells. Previously, Eli Lilly and Company (Lilly) made an initial payment of $60 million to D. E. Shaw Research (DESRES), with potential development and commercial milestone payments of up to $475 million for DES-7114, a lead compound of the Kv1.3-targeted therapeutics program.Recently, the activity of T cells has become a compelling intervention point in IBD[3]. Long-lived memory T-cell populations, particularly tissue resident subsets, may contribute to the chronicity of IBD and represent a potential target for therapy[4]. Kv1.3 expression was elevated in PBMCs from UC patients and correlated with the prevalence of Th1 and Th2 T cells. Kv1.3 expression was also detected on T cells from biopsies of UC patients[5].It is estimated that there are at least 8 million IBD patients worldwide, of which more than 5 million in Europe and the U.S. The global market for autoimmune drugs is projected to reach $140 billion by 2028, with IBD medications accounting for $28 billion, representing 20% of the market share.DPT0218 is a potential ‘best-in-class’ highly selective Kv1.3 inhibitor with a novel scaffold. It has shown positive results in preclinical animal models of various autoimmune diseases such as IBD and AD. The compound exhibits sub-nanomolar potency and has achieved over 2000-fold selectivity for the Kv1.3 channel over a range of other ion channels, including Na+, K+, and Ca2+. It also showed unique DMPK profiles, high colon/ileum concentrations with low systemic exposure in multiple species, demonstrating an excellent safety profile in preclinical studies.“Although antibodies play a pivotal role in the treatment of moderate to severe IBD, their applications are limited by constrained therapeutic efficacy, immunogenicity, parenteral route of drug delivery, poor tolerability, and high cost.” said Xiaomin Zhang, Head of Drug Discovery at DP Technology. “As a small molecule, DPT0218 has shown potent inhibition of T cell activation, proliferation, and cytokine secretion in both in vivo and in vitro studies, demonstrating its potential as an effective treatment for IBD. We’ll actively advance DPT0218 to clinical research and explore its application to other autoimmune diseases.”“DP Technology is the pioneer of AI for Science paradigm, dedicated to integrating ‘AI + Simulation + Experiments’ to address unmet clinical needs.” said Weijie Sun, Founder and CEO of DP Technology. “The dynamics of ion channels are extremely complicated, and our RiDYMO® platform is unequivocally well-suited for the drug development of these highly challenging systems. The successful discovery of DPT0218 is an important step towards achieving the AI for Science vision. We look forward to collaborations with seasoned partners to advance this program to the next milestone.”

The RiDYMO® drug design platform integrates various AI and physical algorithms, dedicated to the development of drugs for “undruggable” targets and “best-in-class” molecules. As one of its core algorithms, Reinforced Dynamics (RiD) has a significant advantage in the sampling efficiency of molecular dynamics simulation. By fully leveraging the high-dimensional representation capabilities of neural networks, RiD can efficiently capture dynamic conformational changes in complicated biomolecular systems.
The RiDYMO® platform is dedicated to studying the dynamics of biological systems and revealing cryptic binding sites, encompassing a range of challenging systems including protein-protein interactions (PPIs), intrinsically disordered proteins (IDPs), membrane proteins, RNA, and others. It has also been validated on “undruggable” targets including c-Myc and GPX4.
About DP TechnologyDP Technology, an “AI for Science” new paradigm-driven company, dedicated to applying Artificial Intelligence and Molecular Simulation algorithms to solve important scientific problems by combining advanced computational methods.Relying on DP Technology’s world-leading Dynamics-based drug design platform, RiDYMO®, and the de novo design platform for macromolecules, the team has established external collaborations and built up strong internal pipeline, focusing on three areas of CNS, oncology and autoimmune diseases.More:https://www.dp.tech/enBusiness:bd@dp.tech
Media:pr@dp.tech
Reference[1] Chiang EY, Li T, Jeet S, et al. Potassium channels Kv1.3 and KCa3.1 co-operatively and compensatorily regulate antigen-specific memory T cell functions. Nat Commun 2017:14644.[2] Lin CS, Boltz RC, Blake JT, et al. Voltage-gated potassium channels regulate calcium-dependent pathways involved in human T lymphocyte activation. J Exp Med 1993:637–45.[3] Sandborn WJ, Su C, Sands BE, et al.; OCTAVE Induction 1, OCTAVE Induction 2, and OCTAVE Sustain Investigators. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017:1723–36.[4] John T. Chang. Pathophysiology of Inflammatory Bowel Diseases. N Engl J Med 2020:2652-64.[5] Anna-Lena Unterweger, Morten Ø. Jensen, Fabrizio Giordanetto, et al. Suppressing Kv1.3 Ion Channel Activity with a Novel Small Molecule Inhibitor Ameliorates Inflammation in a Humanised Mouse Model of Ulcerative Colitis. Journal of Crohn’s and Colitis 2021:1943–58.
推荐关注
关于深势科技
深势科技是“AI for Science”科学研究范式的引领者和践行者,致力于运用人工智能和多尺度的模拟仿真算法,结合先进计算手段求解重要科学问题,为人类文明最基础的生物医药、能源、材料和信息科学与工程研究打造新一代微尺度工业设计和仿真平台。
我们开创性地提出了「多尺度建模+机器学习+高性能计算」的革命性科学研究新范式,并推出了Bohrium®科研云平台、Hermite®药物计算设计平台、RiDYMO®难成药靶标研发平台及 Piloteye®电池设计自动化平台等工业设计与仿真基础设施,颠覆了现有研发模式,打造“计算引导实验、实验优化设计”的全新范式。
深势科技是国家高新技术企业、国家专精特新“小巨人”企业,总部位于北京,并在上海、深圳等城市布局研发中心。科研技术团队由中国科学院院士领衔,汇集了超百位数学、物理、化学、生物、材料、计算机等多个领域的优秀青年科学家和工程师,其中公司的博士及博士后占比超过35%。核心成员获得过2020年全球计算机高性能计算领域的最高奖项“戈登贝尔奖”,相关工作当选2020年中国十大科技进展和全球AI领域十大技术突破。






 ufabet
มีเกมให้เลือกเล่นมากมาย: เกมเดิมพันหลากหลาย ครบทุกค่ายดัง
ufabet
มีเกมให้เลือกเล่นมากมาย: เกมเดิมพันหลากหลาย ครบทุกค่ายดัง


